Dr. Cheng
 |
|
Lin Cheng, M.D., Ph.D.
Investigator, Center for the Prevention and Treatment of Visual Loss
Postdoc Fellow, Department of Ophthalmology & Visual Sciences, University of Iowa
Phone: (319) 335-9565
E-mail: lin-cheng@uiowa.edu
Education
MD, School of Medicine, Chengdu University of Traditional Chinese Medicine
PhD, Clinical Pharmacology, Xiangya Medical School, Central South University
Postdoc Fellow, Ophthalmology, Schepens Eye Research Insititute, Harvard Medical School
Postdoc Fellow, Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University
Research Interests
1) Regeneration of the Trabecular Meshwork
Glaucoma is a leading cause of irreversible blindness throughout the world and the second leading cause of blindness in the US. High intraocular pressure (IOP) is the main reason for most forms of glaucoma. The reduced cellularity of trabecular meshwork (TM) leads to blockade of aqueous humor outflow, thus causing high IOP. Regenerating TM and restore outflow facility may be a promising approach to treat primary open-angle glaucoma. Previously, Dr. Kuehn's laboratory has pioneered methods to replace these lost cells with induced pluripotent stem cells. These cells can be derived from patients and can then be differentiated into the trabecular meshwork cells. Transplanting these cells into the eyes of animal models of glaucoma leads to restoration of normal intraocular pressure. As the key person working on the project of trabecular meshwork regeneration at Kuehn Lab, I am currently working on the mechanisms how stem cells could lead to endogenous TM proliferation.
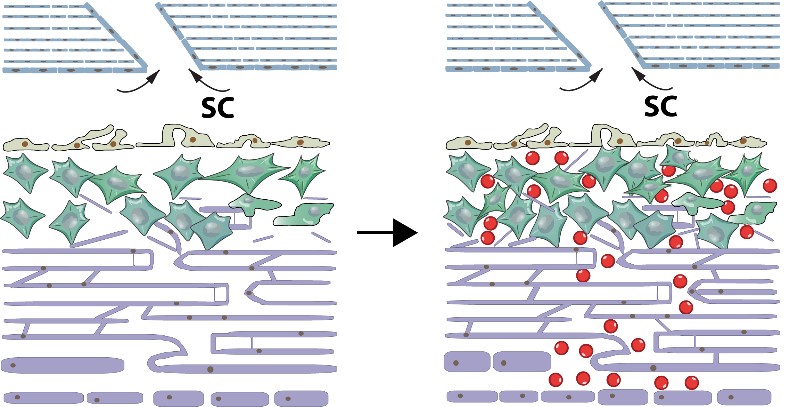
After transplantation of human iPSC-TM (red) cells into donor eyes, the endogenous TM cells proliferate and restore outflow facility.
2) Using retinal and cerebral organoids to study progressive neurodegenerative diseases
The neurodegenerative diseases, including traumatic brain injury (TBI), Alzheimer’s disease, glaucoma, and stroke, are predicted to increase rapidly in the coming decades. Unfortunately, current therapies for most neurodegenerative diseases are symptomatic, and few, if any, disease-modifying strategies are available. The main reason is lack of good model systems to study. With the development of induced pluripotent stem cells (iPSC), we are able to reprogram human fibroblasts or urine epitheliums into pluripotency and then differentiated into different neuronal subtypes or neural organoids. An organoid is a 3D structure grown from stem cells and consisting of organ-specific cell types that self-organizes through cell sorting and spatially restricted lineage commitment. Since iPSC are self-renewable, it can provide us with unlimited sources for disease modeling, engineering, drug screening or autologous cell transplantation. We are currently differentiating human iPSC into retinal and cerebral organoids to investigate the pathogenesis of TBI and glaucoma.
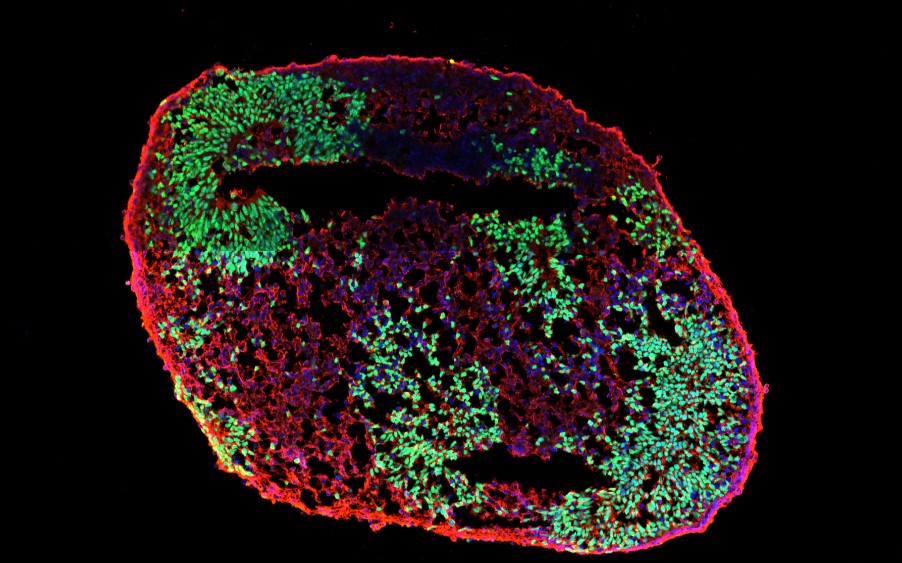
Human iPSC-derived brain organoids, expressing SOX2 (green, a neural progenitor marker) and TUJ1 (red, an early neuron marker).



















